Abstract
Background: Light-chain (AL) amyloidosis is a rare, systemic disease caused by plasma cell dyscrasia (PCD). Excess immunoglobulin light chains misfold and form insoluble amyloid fibrils that deposit in organs, primarily the heart. Survival depends largely on the extent of heart involvement. Current therapies target PCD to halt fibril formation but do not treat existing fibril deposited in organs. CAEL-101 is a novel, investigational, potentially first-in-class therapy designed to remove amyloid fibrils from organs. Safety, tolerability, and biomarker data after all patients with AL amyloidosis enrolled in the trial received 1 year of treatment with CAEL-101, administered initially with cyclophosphamide-bortezomib-dexamethasone (CyBorD) ± daratumumab, are presented.
Methods: Adult patients with AL amyloidosis (European Modification of Mayo Stage I-IIIa) and measurable hematologic disease were eligible for this ongoing, open-label, phase 2 study (NCT04304144). Other forms of amyloidosis and multiple myeloma were excluded. CAEL-101 ≤1000 mg/m2 is administered every other week with anti-PCD therapy as directed by the investigator. In addition to safety assessments, cardiac and renal response were assessed by change over time in N-terminal pro-brain natriuretic peptide (NT-proBNP) and proteinuria, respectively.
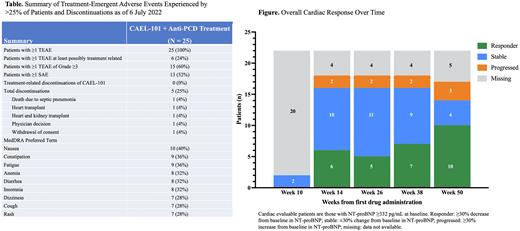
Results: Patients (n = 25; mean age 65 years; 72% male) with Mayo Stage I (8%), II (76%), and IIIa (16%) AL amyloidosis were treated with CAEL-101 for 1 year at this analysis. Patients presented with cardiac involvement (n = 22), renal involvement (n = 9, reported from one site), and prior anti-PCD therapy (n = 20). All 25 patients experienced treatment-emergent adverse events (TEAEs), and 6 (24%) experienced at least possibly treatment-related TEAEs. Five patients discontinued for non-treatment-related reasons (Table). Fifteen (60%) patients experienced TEAEs of Grade ≥3 severity and 13 (52%) experienced ≥1 serious adverse event (SAE). The most common TEAEs were nausea (n = 10), constipation or fatigue (n = 9 each), anemia, insomnia, or diarrhea (n = 8 each), and dizziness, cough, or rash (n = 7 each). After 1 year of CAEL-101 treatment of the 22 cardiac evaluable patients (baseline NT-proBNP ≥332 pg/mL), 10 (46%) patients experienced ≥30% NT-proBNP decrease from baseline, 4 (18%) were stable (±30% change from baseline), and 3 (14%) showed disease progression (≥30% NT-proBNP increase from baseline); data for 5 patients were missing. Of the 9 renal evaluable patients from a single site as reported by the investigator, 8 showed ≥30% decrease from baseline in proteinuria.
Conclusions: The long-term safety evaluation of CAEL-101 continues in this study. All patients currently enrolled have been treated for at least 1 year. At this 1-year time point, CAEL-101 has been generally well tolerated without evidence of organ toxicity. Organ response persisted even after cessation of anti-PCD treatment. Most TEAEs were mild to moderate. A phase 3 program has commenced to elucidate the efficacy and safety of CAEL-101 in cardiac AL amyloidosis European Modification Mayo Stages IIIa and IIIb.
Disclosures
Valent:Alexion, AstraZeneca Rare Disease: Research Funding. Liedtke:Gilead: Research Funding; Natera: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Research Funding; Caelum: Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees; Seagen Inc.: Research Funding; Alnylam: Membership on an entity's Board of Directors or advisory committees; GSK: Membership on an entity's Board of Directors or advisory committees; Kura Oncology: Membership on an entity's Board of Directors or advisory committees; Kite: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Allogene: Research Funding; Adaptive: Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees. Zonder:Alnylam, Amgen, Caelum Biosciences, Celgene, Intellia, Janssen, Prothena, Regeneron: Consultancy; Alexion, AstraZeneca Rare Diseas: Research Funding; Janssen: Research Funding; BMS: Consultancy, Current Employment, Research Funding; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees. Tulchinskiy:Alexion, AstraZeneca Rare Disease: Consultancy. Udata:Alexion, AstraZeneca Rare Disease: Current Employment. Ramirez:Alexion, AstraZeneca Rare Disease: Ended employment in the past 24 months. Szymaniak:Alexion, AstraZeneca Rare Disease: Current Employment. Catini:Alexion, AstraZeneca Rare Disease: Current Employment. Quarta:Alexion, AstraZeneca Rare Disease: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal